A one-stop Integrated Learning Platform where you can Upskill, Grow and Get Placed!
Our Accomplishments

Popular Categories
Explore Featured Courses

Clinical Research Approaches
Research is always necessary when it comes to healthcare since the high-quality treatment. We have access to today as the result of years of arduous work by researchers. The basis for all medical product discovery and development is laid by clinical research. This module will provide you with an understanding of clinical trial management which include, the evolution of clinical research as well as the path a drug takes from the laboratory to the market.

Drug Approval and Marketing Process
Drugs must be tested for safety and efficacy before being made available to consumers. Pharmacovigilance course, will include (but not limited to) the risk response strategies, safety in pharmaceutical industry, various drug kinds and the laws governing their discovery, approval, manufacture, and marketing by reading about the drug approval, marketing procedures and much more. Drugs are not items that a typical customer purchases. There are rules and norms regarding the use of drugs.

Harmonization Activities
The process of establishing uniform standards for the pharmaceutical industry is known as harmonization. Comparing clinical trial data is challenging since different countries conduct clinical studies using different protocols. This module's advanced principles help you comprehend why it's important to standardize the clinical data management process internationally. Eliminating needless delays in the global development and accessibility of new medications is the primary goal of this approach. Standard operating procedures in clinical trials and laid protocol helps in this direction.

Scientific Community - Roles & Responsibilities
The ethical and regulatory compliance of human trials is contingent upon the efforts of all research participants, each of whom is a key player in the process. The primary players in the research team are described in detail in this module of clinical research & regulatory affairs. By comprehending this subject, you will be able to apply for jobs in marketing, regulatory affairs, and pharmacovigilance in the pharmaceutical research and development business.

Clinical Trial Documents
In any clinical trial proper collection and storage of study data and other relevant clinical trial information is vital for accurate records. The trial documentation is a legal requirement which is relevant to all trials. It helps to reconstruct the trial as it happened and to evaluate the trial. This module will give you a thorough understanding of good documentation practices which will help to ensure the success of the study at the clinical trial site.

Clinical Trial Protocol & Design
It is crucial to examine and critically assess the study's results using a well-written clinical trial protocol and a well-defined clinical trial design in which the entire clinical trial process is succinctly summarized. A series of protocols or actions that must be taken in order to complete the clinical study are outlined in this module. The significance of comprehending the procedures involved in creating a clinical research/clinical trial protocol is highlighted in this session as a means of conducting a suitable study and producing accurate findings.

Regulatory Authorities World-Wide
To guarantee the efficacy and safety of pharmaceuticals, clinical trials must be controlled. Every nation has an organization under its own government that oversees healthcare products. Regulatory agencies have a crucial role in ensuring the quality, safety, and efficacy of medications that are made available to the general population. Regulatory affairs certification is mandatory to market a pharma formulation. Completing this module will enable students to understand how regulatory agencies including the agency involved in drug regulatory affairs contribute to the public's access to safer and more effective medications

Clinical Data Management
Clinical Data Management (CDM) is a vital stage in clinical research protocol that results in high-quality, reliable, and statistically sound data from clinical trials. This course thoroughly explains the process involved in CDM that includes Case Report Form (CRF) design, database design, data entry and validation, discrepancy management, medical coding, database reconciliation, query creation, and database locking procedures. This course is designed to teach life science/health science graduates how to manage and report huge amounts of clinical data technically and scientifically and finally, database reconciliation. This understanding of various CDM processes will undoubtedly assist learners in working with various CDM software provided by various organizations.

Pharmacovigilance
Without Pharmacovigilance, clinical research cannot be accomplished since drug safety monitoring is the foundation for keeping a society safe and healthy. Pharmacovigilance is a scientific discipline that focuses on detecting, validating, quantifying, assessing, and improving drug safety. The key activities in Pharmacovigilance include delivering end-to-end medical, safety, and analytical services such as medical review, causality assessment, case narratives and processing, and adverse drug reaction prevention with the involvement of regulatory authority. The Pharmacovigilance course covers all of these topics, with an emphasis on reporting systems and various ADR forms, causality assessment, case narration, risk management plans, and various pharmacovigilance programs. This course will provide you a solid understanding of the pharmacovigilance process and operations. Our curriculum is completely industry‑specific and appropriate to applicants from both within and outside the pharmaceutical industry who want to be a part of the pharmacovigilance sector.

Good Clinical Practices
Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting clinical trials involving human subjects. It aims to ensure the protection of participants' rights, safety, and well-being, as well as the credibility and reliability of trial results. This course helps the learners to understand the principles of clinical practices and its application in clinical trials.
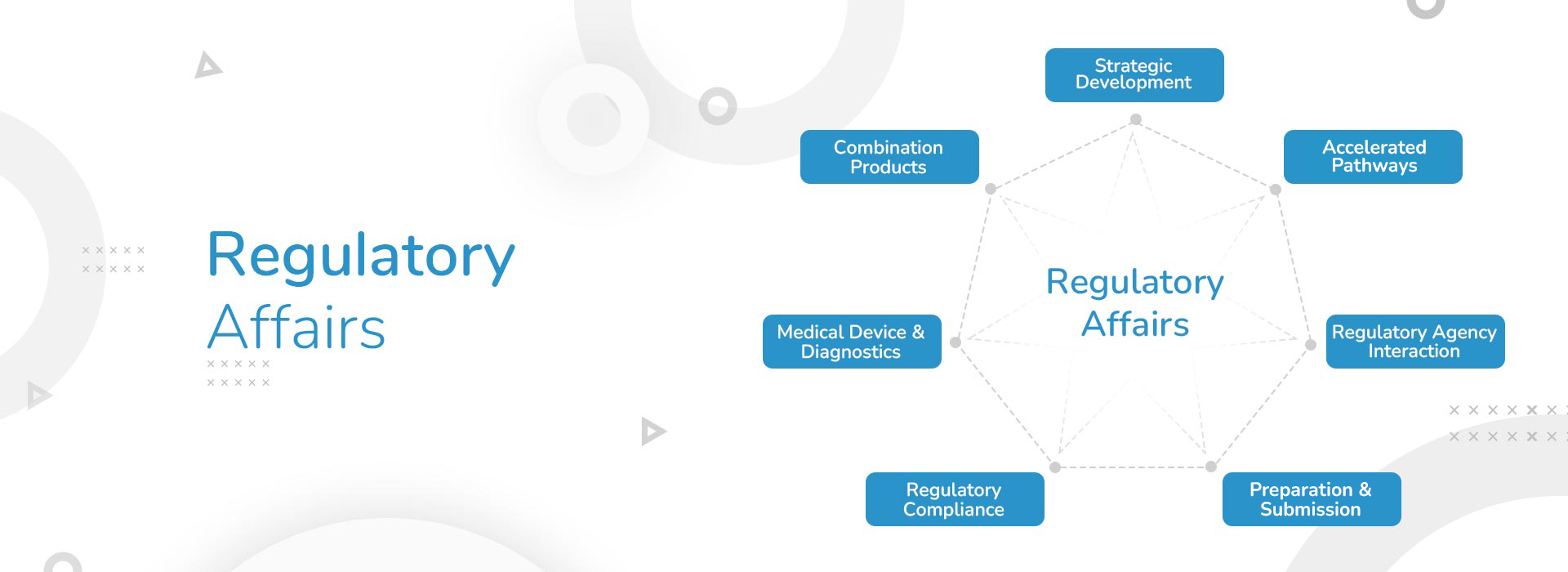
Regulatory Affairs
Regulatory Affairs courses deals with the regulatory requirements necessary for the development, approval, and marketing of pharmaceuticals, medical devices, biologics, and other health-related products. This course in Regulatory Affairs cover a broad range of topics to equip professionals with the knowledge and skills needed to navigate the complex regulatory landscape.

Narrative Writing
This course in narrative writing typically covers the skills and knowledge necessary to effectively communicate adverse drug reactions (ADRs) and other safety-related information in a clear and comprehensive manner in healthcare communication. It aims to equip participants with narrative competence necessary to document and communicate safety information accurately and comprehensively. The narrative writing skill is crucial for maintaining public safety, supporting regulatory compliance, and contributing to the ongoing monitoring of the safety profile of pharmaceutical products.

Medical Coding
Medical coding classes in this course aims to equip students with the skills and knowledge needed to accurately code medical information in a standardized manner. This proficiency is crucial for maintaining consistency in adverse event reporting, supporting regulatory compliance, and facilitating communication within the pharmaceutical and healthcare industries.
Interpreting Dental Radiographs
Dental radiographs are like our eyes. Just like our eyes are the window to the outside world, radiographs provide an aperture to the oral cavity. They give an in-depth knowledge of the condition of the teeth, gums, bones, and sinuses. The discovery of oral radiology changed the face of Dentistry. The images created on the radiograph, along with the clinical examination and case history, help the dentist give the patients the right care and treatment. Interpreting the radiographs is crucial in detecting diseases and abnormalities of the oral cavity. In dental practice, when confronted with a lesion or swelling in the oral cavity, a dentist becomes perplexed. Starting the treatment without a radiograph and incomplete history of the lesion is inappropriate and not fair to the patient. Sometimes a single radiograph may not suffice, multiple radiographs may be the key to the right treatment. Some conditions look similar, for example, a cyst and an abscess. Both present as a “mass” or “swelling’. Differentiating or judging between a cyst and an abscess is not possible with just a physical examination, even for experienced dentists. A detailed radiographic interpretation and histological report will give a definite answer.

Sports Nutrition
Sports nutrition is a multifaceted discipline that encompasses various factors essential for optimizing athletic performance and maintaining overall health. It involves tailoring dietary intake to meet the specific needs of athletes based on factors such as their sport, training regimen, body composition goals, and individual physiology. By prioritizing physical fitness, consuming a balanced diet rich in macronutrients and micronutrients, using supplements judiciously, and adhering to anti-doping regulations, athletes can maximize their potential and achieve their goals safely and ethically.

Communication Skills
Most of us spend a great deal of time outside the comfort of our home and familiar surroundings to study or work. For many of us, getting introduced to new surroundings and different people may seem uncomfortable and stressful. Learning to communicate well takes care of most of our problems. Effective communication skills help us navigate through difficult situations. At the workplace, to perform the duties that our job profile demands, we need the support of a whole team of people that work with us. Some of us have to build business relationships outside our current place of work. Interpersonal skills may not come easily to many of us. Confronted with a stressful situation at work, how can we manage to figure out a solution that works for us? Is it possible to maintain a good work-life balance without our personal and professional lives merging and interfering with each other? Good communication skills promote good relationships in the workplace as well as in personal life. Our course offering on Communication Skills or Soft Skills gives an in-depth analysis of prominent emotional and interpersonal challenges that an individual struggles with in everyday work life. We offer solutions to help improve people’s ability to express themselves well. Our Communication Skills course will help you to enhance your verbal and non-verbal communication skills. Convey information effectively, understand others, and build strong relationships. Improve your ability to connect with others, resolve conflicts and handle stress in your life efficiently.
Our Webinars
Students' Testimonials
Talking about placements, here's what our dear students have to say about their own experiences with True Lessons.




































